THE NIGHTSHIFT MODEL — SCIENCE ANNEX Supporting References, Mechanistic Notes & Foundational

### THE NIGHTSHIFT MODEL — SCIENCE ANNEX (Revised)
Supporting References, Mechanistic Notes & Foundational Evidence
November 25, 2025 — Revised for Accuracy
**TABLE OF CONTENTS**
1. Terrain Collapse & The Mucus Biome
2. Choline Cycling & Acetylcholine Deficit
3. Vagus Nerve / HRV / Autonomic Collapse
4. Sleep Architecture & N3 Fragmentation
5. Glymphatic System & Meningeal Lymphatics
6. Microglia: Priming & Plasticity
7. Ferroptosis, Iron Dysregulation & Regional Vulnerability
8. Lifespan Spectrum Associations
9. Paternal & Maternal Epigenetic Influences
10. Research Roadmap & Predictions
11. Diagrams (ASCII Models)
**1. TERRAIN COLLAPSE & THE MUCUS BIOME**
Depletion of key butyrate-producing taxa (Akkermansia, Faecalibacterium, Roseburia) linked to modern exposures (antibiotics, diet, glyphosate).
Key References:
1. Zhang T et al. Akkermansia muciniphila improves barrier function. Front Microbiol 2019. DOI: 10.3389/fmicb.2019.02255
2. Sokol H et al. Faecalibacterium prausnitzii as anti-inflammatory commensal. PNAS 2008. DOI: 10.1073/pnas.0804812105
3. Sonnenburg ED & JL. Microbial diversity loss in industrialized nations. Cell 2019 (review of erosion).
**2. CHOLINE CYCLING & ACETYLCHOLINE DEFICIT**
Microbial regulation of choline metabolism; deficits observed across neurodevelopmental/neurodegenerative conditions.
Key References:
4. Tang WHW et al. Gut microbiota-dependent TMAO from choline. NEJM 2013. DOI: 10.1056/NEJMoa1109400
5. Blusztajn JK et al. Choline in neurological function. Annu Rev Nutr 1999 (updated reviews link to deficits in ASD/AD).
**3. VAGUS NERVE, HRV & AUTONOMIC COLLAPSE**
Low vagal tone common across ASD, ADHD, schizophrenia, PD, AD.
Key References:
6. Tracey KJ. Cholinergic anti-inflammatory pathway. Nature 2002. DOI: 10.1038/nature01303
7. Neuhaus E et al. Reduced vagal tone in ASD. J Autism Dev Disord 2016.
**4. SLEEP ARCHITECTURE & N3 FRAGMENTATION**
Reduced slow-wave sleep in multiple disorders; critical for glymphatic activation.
Key References:
8. Hablitz LM et al. Glymphatic function peaks in NREM sleep. Nat Commun 2019. DOI: 10.1038/s41467-019-13995-3
9. Maski K et al. Sleep disturbances in ASD. Neurology 2018.
**5. GLYMPHATIC SYSTEM & MENINGEAL LYMPHATICS**
Sleep-dependent waste clearance; impairment linked to protein aggregation.
Key References:
10. Iliff JJ et al. Paravascular CSF-ISF exchange (glymphatic discovery). Sci Transl Med 2012. DOI: 10.1126/scitranslmed.3003748
11. Louveau A et al. Meningeal lymphatics. Nature 2015. DOI: 10.1038/nature14432
12. Nedergaard M & Goldman SA. Glymphatic failure as waste clearance hypothesis. Nat Rev Neurosci 2020 (review).
**6. MICROGLIA: PRIMING & PLASTICITY**
Prenatal priming leads to persistent activation; role in synaptic pruning.
Key References:
13. Paolicelli RC et al. Microglial synaptic pruning. Science 2011.
14. Bilbo SD et al. Early-life immune programming of microglia. Brain Behav Immun 2018.
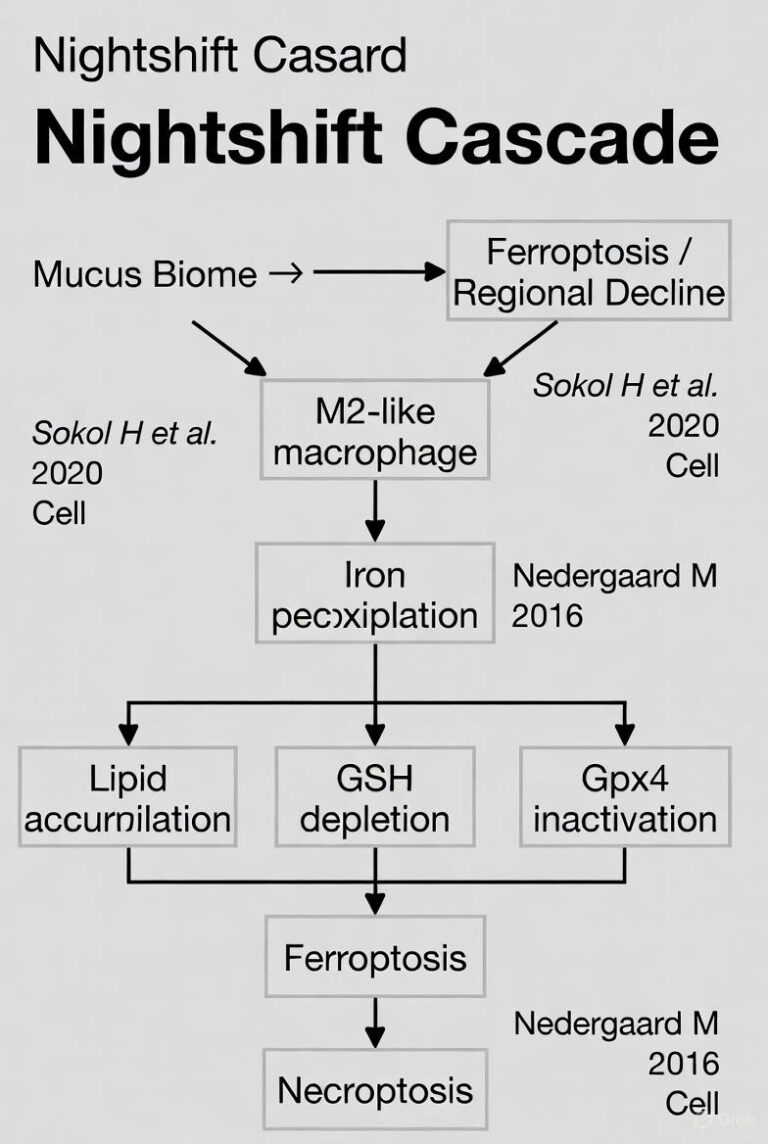
**7. FERROPTOSIS, IRON DYSREGULATION & REGIONAL VULNERABILITY**
Iron-lipid peroxidation pathway implicated in PD and other disorders.
Key References:
15. Stockwell BR et al. Ferroptosis mechanism and disease link. Cell 2017. DOI: 10.1016/j.cell.2017.09.044
16. Dixon SJ et al. Ferroptosis in disease. Nat Rev Dis Primers 2023 (links to neurodegeneration).
**8. LIFESPAN SPECTRUM ASSOCIATIONS**
Shared pathways (inflammation, clearance failure) across ages/disorders.
Key References:
17. Cryan JF et al. Gut-brain axis in neurodevelopment/degeneration. Physiol Rev 2019 (transdiagnostic links).
**9. PATERNAL & MATERNAL EPIGENETIC INFLUENCES**
Sperm small RNAs and maternal inflammation affect offspring brain development.
Key References:
18. Chen Q et al. Sperm tsRNAs in intergenerational inheritance. Science 2016. DOI: 10.1126/science.aad7977
19. Gapp K et al. Paternal stress/epigenetic transmission. Nat Rev Endocrinol 2021 (review).
**10. RESEARCH ROADMAP**
Testable: Longitudinal microbiome-glymphatic-HRV cohorts; preconception interventions; glymphatic enhancement trials.
**11. DIAGRAMS**
A. CASCADE OVERVIEW
“`
Modern Exposures → Mucus Biome Depletion → Butyrate/Choline ↓ → Vagal Tone ↓ → N3 Sleep Fragmentation → Glymphatic Impairment → Waste Accumulation → Microglial Activation → Regional Vulnerability
“`
B. LIFESPAN ASSOCIATIONS
“`
Early Life: Cortical/Prefrontal (ASD/ADHD links)
Mid-Life: Limbic/Nigral (Psychosis/PD)
Late Life: Hippocampal/Cortical (AD)
“`